Zhejiang Poly Pharmaceutical is a wholly-owned subsidiary of Hainan Poly Pharmaceutical (stock code: 300630). In line with the company's strategy to establish international production capabilities, the eye drop workshop has been constructed to meet European Union, U.S. FDA, and Chinese GMP requirements. It is equipped with world-class domestic and international equipment and automated production lines, with an annual production capacity of up to 70 million bottles (calculated at 5ml per bottle).

EMS/BMS Automatic Warning Adjustment System of Differential Pressure, Temperature and Humidity
Compounding Systems are Adaptable to Various Process Requirements:
The compounding system consists of one 300L mixing tank (minimum mixing volume: 35kg) and two 1000L mixing tanks (minimum mixing volume: 80kg). There are three independent compounding systems, all made of 316L stainless steel, capable of meeting batch requirements at different stages, from laboratory scale and pilot-scale to commercial production.

The 300L mixing tank is equipped with a Milipore magnetic stirrer, while the 1000L mixing tank combines Milipore magnetic and mechanical stirring, making it suitable for dissolving complex or slightly soluble substances.
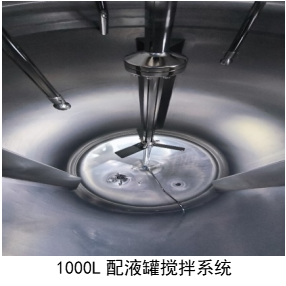

The compounding system includes various utility media such as steam, cooling water, and standalone hot and cold integrated unit (TCU). This TCU can realize different cooling rates of the jacket, with a temperature control accuracy of ±1°C. It also has an independent nitrogen system with a purity level of 99.999%, capable of meeting nitrogen charging requirements such as top-filling and bottom-filling, catering to the production process of oxygen-sensitive products.
The compounding system is equipped with an automatic SIP system and an independent automatic CIP station, facilitating various cleaning methods like acid washing, alkali washing, and cleaning with special detergents, ensuring compliance with specific cleanliness requirements for different products.

Automatic CIP System
Fully automated filling lines meet various specifications and process requirements for production:
The fully automated filling line is imported from Germany ROTA, which can switch molds to produce multiple dose specifications in the range of 5-30ml. Currently, it is equipped with 5ml, 10ml, and 15ml molds.

Fully Automated Filling Line of Germany ROTA
To effectively control the introduction and generation of visible particles, the filling line employs ready-to-use packaging materials, enabling automated production through automatic loading. Additionally, the filling line is equipped with a fully automatic bottle blower, which reduces the risk of particles carried by packaging materials through negative ion wind blowing and simultaneous vacuum suction.

Fully Automatic Bottle Blower
The filling machine is equipped with German-imported peristaltic pumps and online weighing, maintaining filling accuracy within ±3%.
The filling machine features torque feedback.
The tightening torque can be accurately set above 40N.m, and the accuracy can be controlled in the range of ±10N.m
It also has torque overrun alarms and cap placement alarms to ensure product seal integrity. Furthermore, it includes an offline fully automatic torque tester to meet offline sampling requirements. And fixed torque arm reduces measurement errors caused by human factors, meeting European and American standards.
Serialized coding lines meet the coding demands of domestic and international products.
International Product Coding Production Line
At present, Zhejiang Poly Pharmaceutical has a number of eye drop varieties declared in China, the United States, Europe.
Zhejiang Poly Pharmaceutical possesses the global excellent organization system and capability for formulation development, industrial transformation, and production. It maintains a comprehensive quality management system and a professional registration team familiar with multi-country submissions, having successfully obtained GMP certification in China, the European Union, and the United States on multiple occasions.
Welcome domestic and international friends and customers to discuss CDMO/CMO business for mutual development.
Welcome for enquiry, cooperation!
Contact: Mr. Zhou
Email:zhoujun@hnpoly.com

 Home
Home

